Highlights: Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Quis ipsum suspendisse ultrices gravida. Read more
Unlock the Potential of Advanced Nanotechnologies
What We Do
At LamdaGen, we are committed to reshaping the field of advanced plasmonics by delivering innovative biodetection solutions tailored to meet the critical demands of various industries. Our plasmonic technologies enable the creation of simple, advanced and cost-effective detection systems that redefine speed and precision. Our robust and adaptable plasmonic biosensors support a range of applications, including human and animal health, food and water safety, biothreat detection, and environmental monitoring.
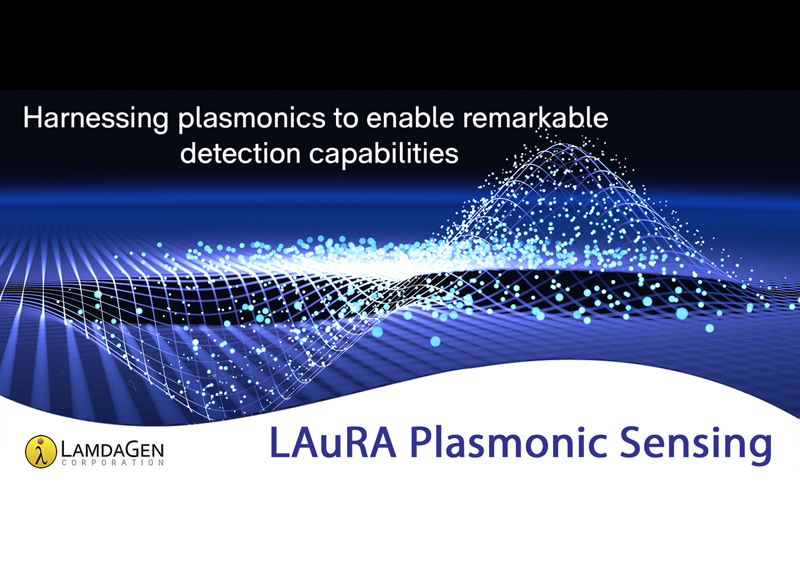
The LAuRA™ platform, powered by LamdaGen’s Plasmonic Amplification Technology™ (PAT™), exemplifies our commitment to enhancing diagnostic and detection capabilities. We utilize our well-established plasmonic systems to improve the sensitivity and precision of diagnostic tools, directly supporting applications in health, safety and monitoring sectors.
We pride ourselves on fostering close collaborations with our partners and clients, ensuring the seamless integration of our plasmonic technologies into their research, development, services and products. Through a commitment to technological excellence and practical application, LamdaGen utilizes a licensing model to distribute our advanced plasmonic detection solutions across varied sectors.
LAuRA™ Plasmonic Sensing, powered by PAT™

Fast Results

High Sensitivity

Patented Technology

Easy to Integrate

Easy to Use

LAuRA Rapid Test System
LAuRA (LamdaGen Au (gold) Rapid Assay) offers a turnkey plasmonic biosensing solution, combining the speed and convenience of lateral flow assays with the high sensitivity and quantitation of ELISA tests.
The LAuRA Rapid Test System delivers laboratory quality results equivalent to gold standard ELISA’s in minutes rather than hours. LAuRA’s test procedure is simple and easy to use. Disposable cartridges can be designed to run single or multi-target tests and deliver results 15 minutes or less.
Leveraging the inherent properties of advanced nano-based plasmonics, the LAuRA immuno-based platform provides high sensitivity detection capabilities far exceeding those of existing rapid assays. The patented platform is flexible and scalable, enabling uniquely powerful detection systems ranging from small benchtops to handhelds to throughput.
Innovative LAuRA systems are being developed for applications in Human Health, Food & Water Safety, Biothreat Detection, and Animal Health.
What would you like to learn?
Latest News


Interested in our biosensors & collaborative opportunities?
Contact us to explore how our detection technologies can benefit your R&D, Services and Product objectives.
Let’s discuss possibilities and drive innovation together.


